Laboratory of algal biotechnology
Kumar Saurav´s group
Natural products: Biosynthesis and regulation
Natural products: Biosynthesis and regulation
We focus on the research of cyanobacterial secondary metabolites with potential use in disease prevention in humans, livestock, and the underlying mechanisms of their biosynthesis using various techniques of molecular biology. By combining modern chemical techniques with bioinformatic and molecular methods, we are able to comprehensively study the compounds starting with their isolation following bioactivity-guided purification and the following detailed characterization of their properties, functions, and biosynthetic pathways. The main goal of our research is to indentify novel compounds with antimicrobial properties to help counteract the current shortage of effective antibacterial drugs.
Quorum Sensing Inhibitory Compounds from Cyanobacteria
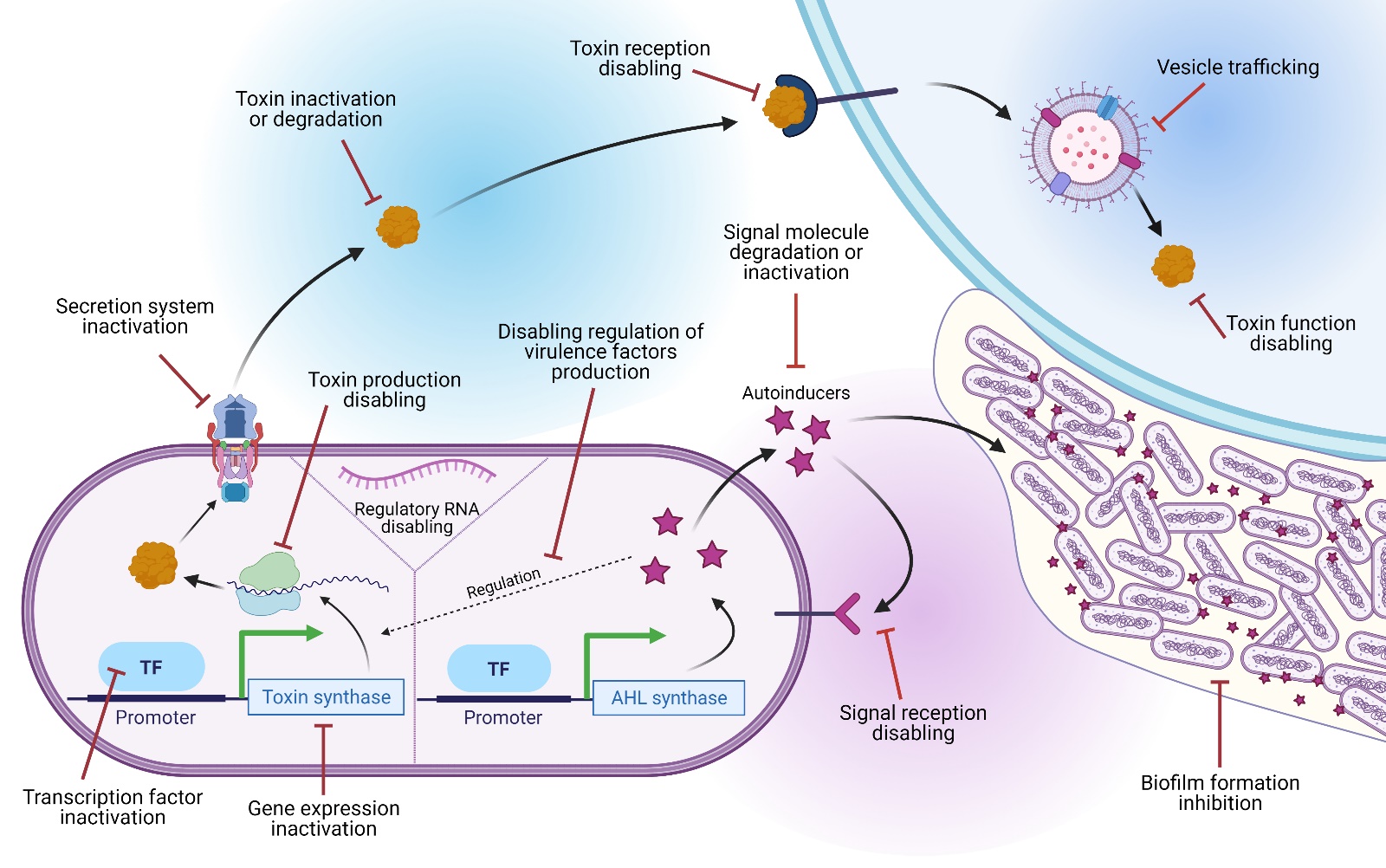
Quorum Sensing (QS) is a type of bacterial communication mediated by small molecules called autoinducers (AI). With help of autoinducers, bacteria are able to monitor the density of their population and regulate their gene expression accordingly. Our group focuses on identifying QS inhibitory molecules produced by cyanobacteria, which may help alleviate the current shortage of antibiotics effective against multidrug-resistant bacteria. By taking the leverage of QS inhibitory compounds, we also try to find alternative drug therapies.
Role of epibionts in regulating production of Cyanopeptides in Cyanobacteria
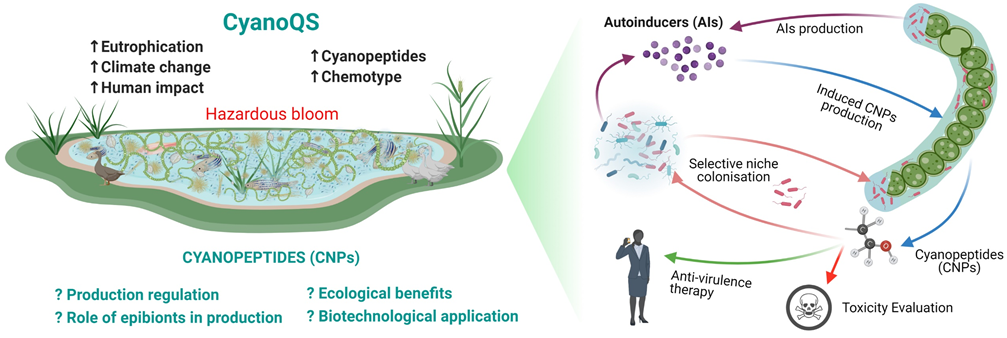
Cyanobacteria host microbes often living within their mucilage envelope, which makes the task onerous to separate these partners that are naturally bound to each other. Although the primary function of heterotrophic bacteria is decomposition, it is now accepted that some bacteria can also play a part in host growth promotion and establishing mutualistic interactions. This paradoxical dual function often complicates such studies on interactions, requiring a complex study on the complete set of ecological functions of each partner. Interspecies interactions among the biofilm-forming cyanobacteria and their epibionts have been neglected even though they might underlie metabolite production as well as biofilm development. It is also assumed that for sustained colonisation by epibionts on cyanobacteria with specific taxa, an intricate mechanism must be involved with localised specificity for survival and maintenance of its biota. We try to make use of two complementary phenomena (QS and QS Inhibition), connected via a regulatory mechanism, towards cyanopeptides production and discovery of alternative drug therapies.
Lasso peptides in cyanobacteria
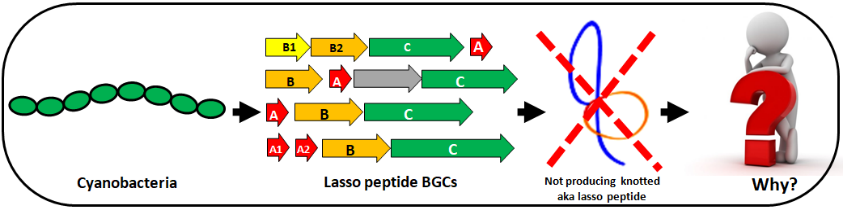 Lasso-peptides (LPs) are a class of ribosomally synthesized and post-translationally modified (RiPPs) natural products. They have emerged as drug discovery candidates due to their unique lasso-like topology and diverse biological activity. The rise of microbial genome sequencing and genome mining has provided the opportunity to discover and exploit these otherwise inaccessible LPs for drug discovery. Direct pathway cloning and heterologous expression of RiPP gene clusters from microbial genomes is an effective strategy to access encoded peptides and produce novel derivatives with potentially enhanced bioactivity. Although cyanobacteria are considered a prolific source of natural products, including RiPPs, LPs have remained uninvestigated in these organisms so far. So far LPs are naturally endowed with a broad spectrum of activity. LPs also possess function as potent receptor antagonists, anti-HIV activity at micromolar concentration and receptor antagonism activity as well as found to be an antiviral agent.
Lasso-peptides (LPs) are a class of ribosomally synthesized and post-translationally modified (RiPPs) natural products. They have emerged as drug discovery candidates due to their unique lasso-like topology and diverse biological activity. The rise of microbial genome sequencing and genome mining has provided the opportunity to discover and exploit these otherwise inaccessible LPs for drug discovery. Direct pathway cloning and heterologous expression of RiPP gene clusters from microbial genomes is an effective strategy to access encoded peptides and produce novel derivatives with potentially enhanced bioactivity. Although cyanobacteria are considered a prolific source of natural products, including RiPPs, LPs have remained uninvestigated in these organisms so far. So far LPs are naturally endowed with a broad spectrum of activity. LPs also possess function as potent receptor antagonists, anti-HIV activity at micromolar concentration and receptor antagonism activity as well as found to be an antiviral agent.
If you are interested in these topics, please contact: Dr. Kumar Saurav (saurav@alga.cz, +420 384 340 469) or Dr. Subhasih Saha (saha@alga.cz, +420 384 340 472).