Laboratory of Photosynthesis
Radek Kana`s group
Membranes heterogeneity in photosynthesis
Group members |
 |
| Group picture (Bára Šedivá, Myriam Canonico, Eliška Trsková, Radek Kaňa, Aurelie Crepin, Greg Konert), year 2020 |
Research topics
We study organization of thylakoid membrane proteins into MICRODOMAINS and mechanisms of photoprotection (non-photochemical quenching, NPQ) in photothrophs.
(1) Heterogeneous mosaic of proteins in thylakoids of cyanobacteria - MICRODOMAINS |
|||
|
Biological membranes were originally described as uniform fluid-mosaic of lipids and proteins. Later, heterogeneous membrane areas have been found in many membrane systems including cyanobacterial thylakoids. There, the heterogeneous areas are formed by pigment-protein complexes (photosystems, light-harvesting antennas) responsible for bioenergetics of light-harvesting in photosynthesis. These complexes are naturally self-organized into homo-/hetero- oligomers (nanodomains) that create sub-micrometer-sized in vivo microdomains (e.g. granal/stromal thylakoids in higher plants or cyanobacterial microdomains). We study importance of thylakoid membranes heterogeneity, especially for light-harvesting in cyanobacteria. We use up-to-date methods of superresolution microscopy (Zeiss LSM 880 with Airyscan detector) and time/spatial cross-correlation microcopy (FRAP, FCS etc.) to address microdomains heterogeneity caused by nanodomains. Thanks to the molecular biology methods (specific mutants) we want to establish a link between membrane heterogeneity and the ligh-harvesting function of photosystems and phycobilisoms in vivo. |
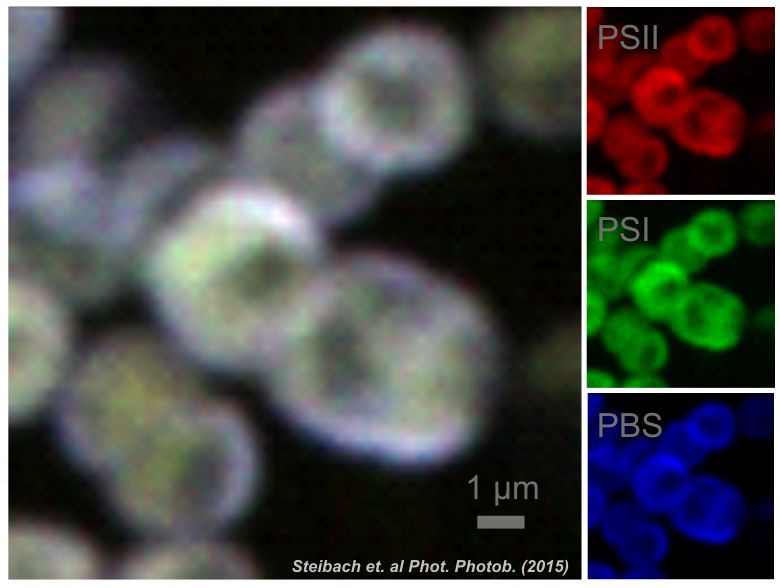
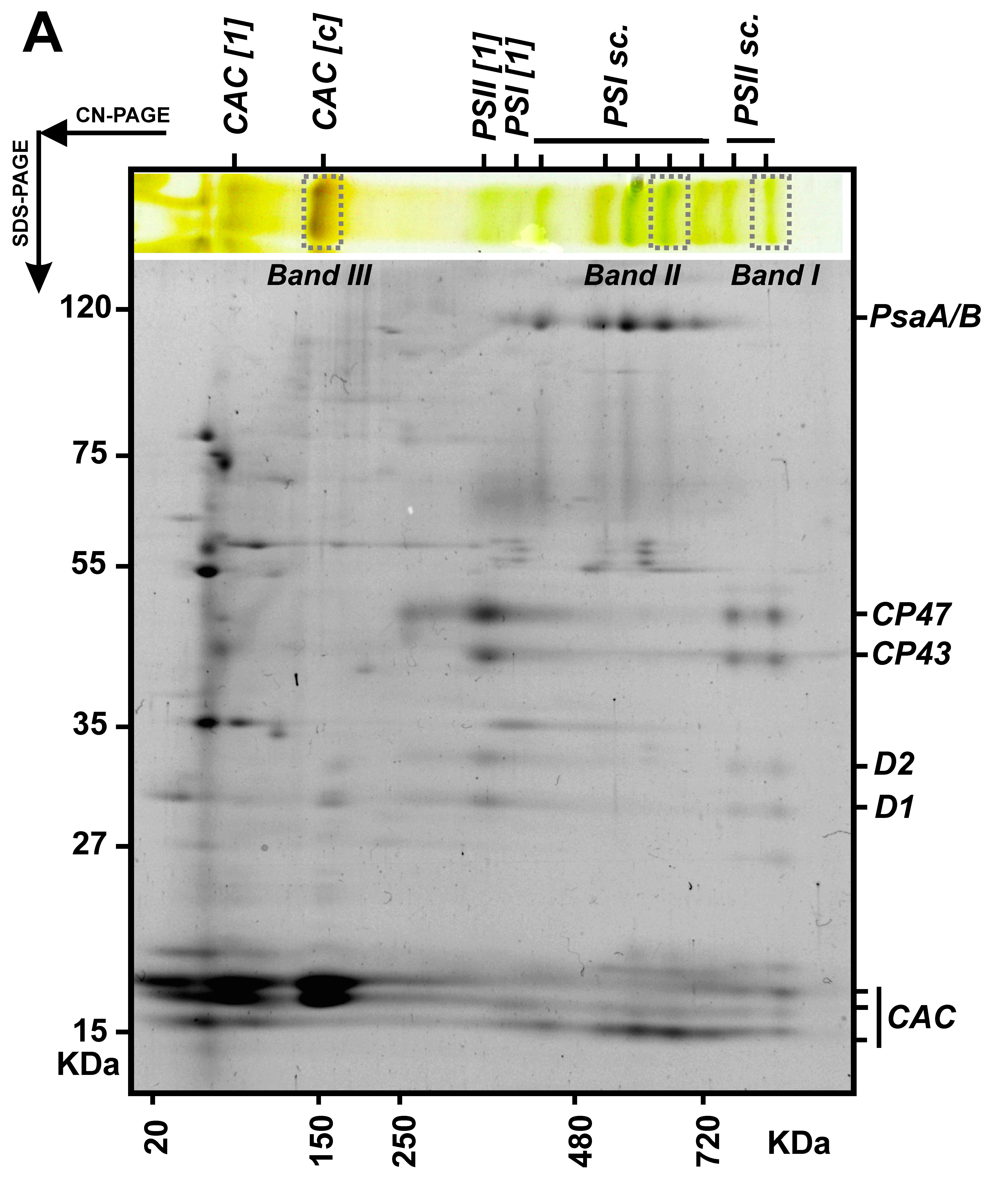
Localisation of main thylakoid membrane protein complexes in cyanobacteria Anabaena sp. PCC 7120 (see Steinbach et al. 2015) |
||
|
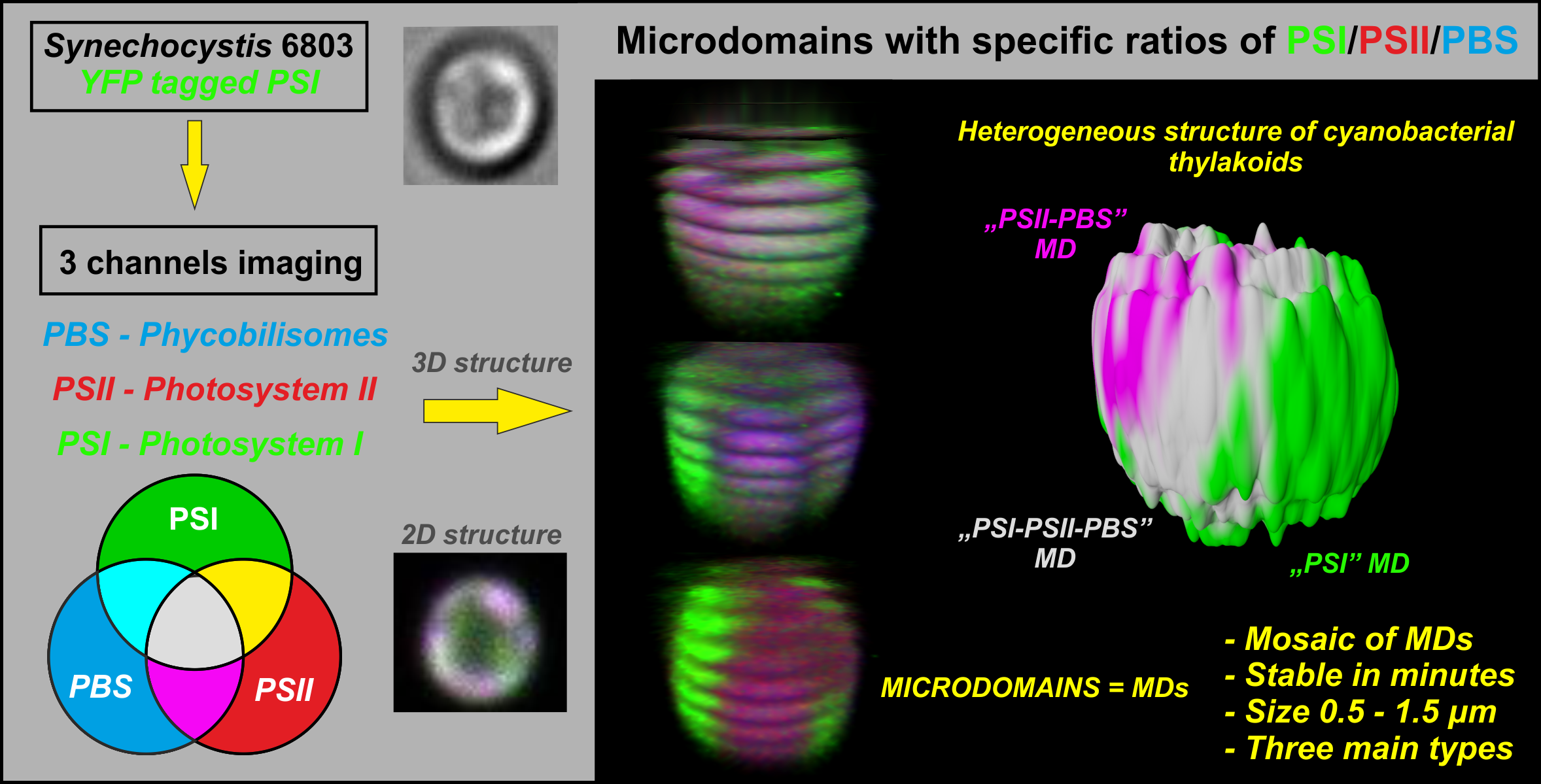
Proposed organization of thylakoid membrane complexes (Photosystem I, Photosystem II and Phycobilisomes) in cyanobacterial thylakoids (see Strašková et al. 2019) |
We have identified and described heterogeneous organization of cyanobacterial thylakoid membrane proteins into microdomains (Strašková et al. 2019; Konert et al. 2019). We study importance of the mosaic-like organization of photosystems (PSI and PSII) and phycobilisomes (PBS) for overall function of photosynthesis and especially for light-harvesting. The microdomain organization of thylakoids is based on PSI/PSII/PBS co-localization (visible as Red-Green-Blue color coding pictures), its is stable in minutes/hours, and it can only slowly adapt to different growing conditions (Canonico et al. 2020). Microdomains seem to be specific for cyanobacterial strains (Steinbach et al. 2015) and they resemble grana/stromal heterogenity in PSI/PSII distribution typical for higher plants thylakoids. |
||
| ______________________________________________________________________________________________________________ | |||
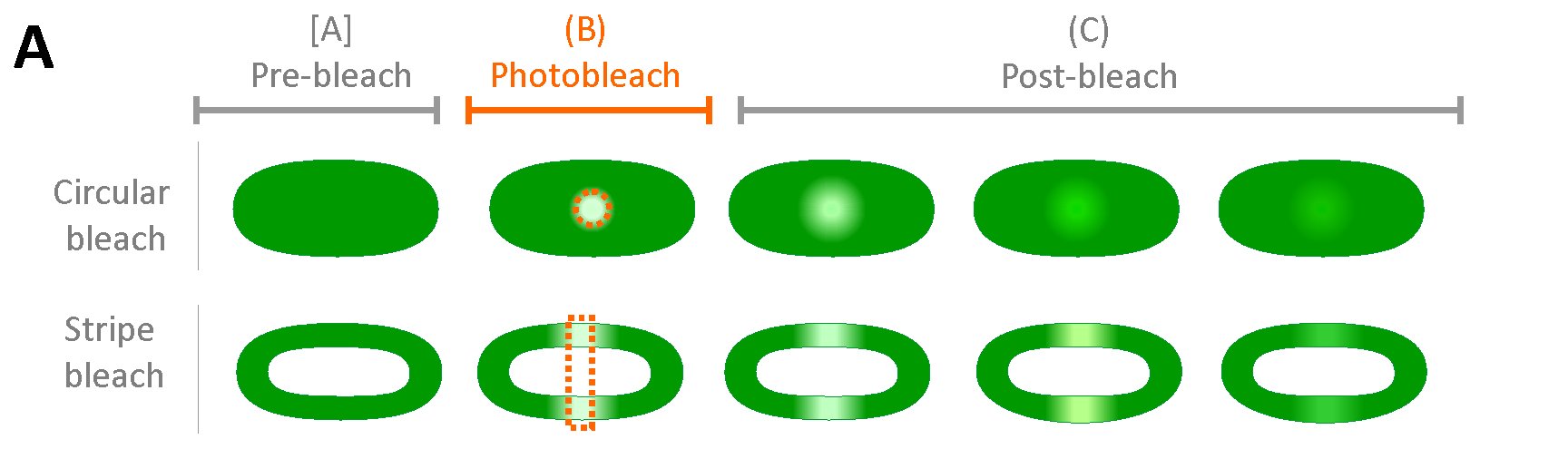
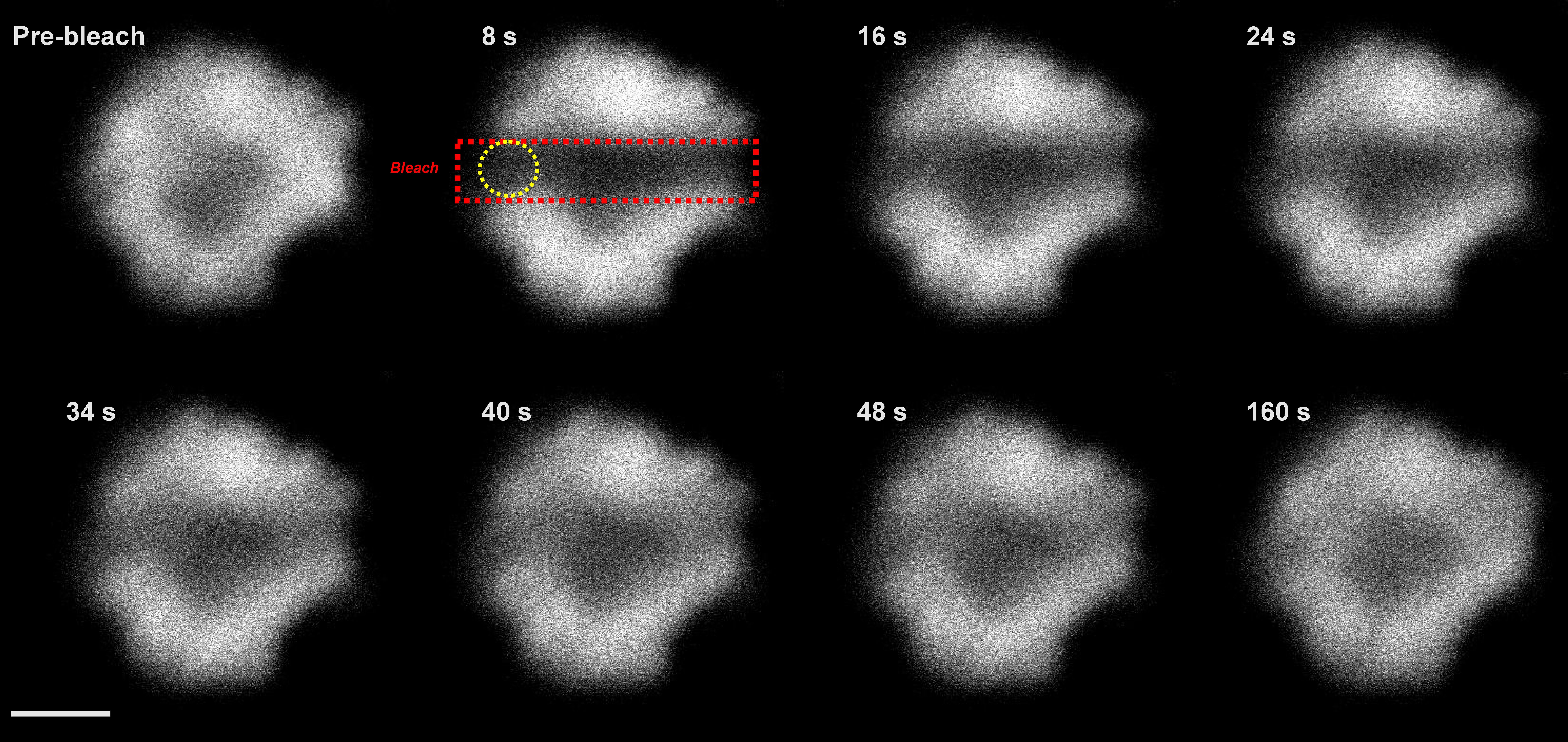
(2) Protein mobilityThylakoid is a higly crowded membrane formed from more then 70% by proteins. This results in limited diffusion of proteins within the thylakoid membrane (see Kaňa 2013). We try to explore factors affecting immobility of photosynthetic proteins and how it is reflected in effeciency of light-harvesting (Kaňa et al. 2014). The protein mobility is explored in situ on single cell level by confocal microscopy methods including FRAP (Fluorescence Recovery After Photobleaching) and FCS (Fluorescence Correlation Spectroscopy). We want to address interconnection between thylakoid membrane structure (in a sence of proteins organization), protein dynamic and their function in photosynthesis. |
Typical setup of FRAP method (for details, see e.g. Kaňa 2013). Typical FRAP image series for PBilisome fluorescence emissiotn (for details, see e.g. Kaňa et al. 2014). |
||
(3) Mechanisms of photoprotection - NON-PHOTOCHEMICAL QUENCHING |
|||
|
Photosynthetic organisms are often exposed to highly fluctuating light-conditions with short periods of excessive irradiation. Therefore, they had to developed various photoprotective mechanisms that can cope with conditions when excessive light can produced ROS and destroy photosynthetic apparatus. We have studied several model organisms including green plants (spinach, Arabidopsis), algae (chromerids, cryptophytes) and cyanobacteria to resolve molecular mechanisms of photoprotection. It includes non-photochemical quenching, photoinhibition, phycobilisome decoupling or state transitions. It is still an open question to what extent these processes are associated with short- or long-term reorganization of TM at the nano- and micro-level. Non-photochemical quenching (NPQ) represents a photoprotective mechanisms dissipating excessive irradiation into heat (see Kaňa and Vass 2008). It is a preciselly controlled process affecting the energy dissipation in the reaction centre (e.g. in extremophilic red algae, see Krupnik et al. 2013) or more often in light-harvesting antennae (see e.g. Kaňa et al. 2012). |
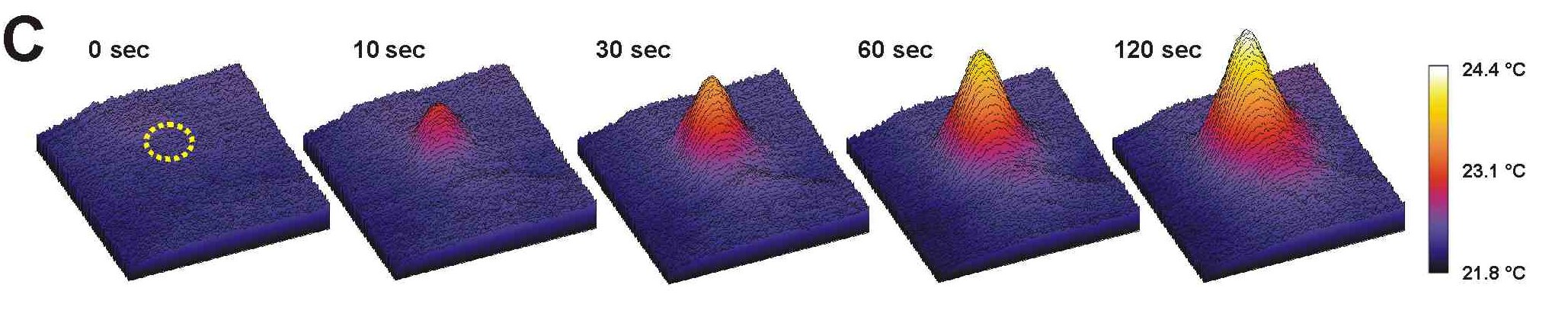
Slow temperature increase induced by irradiation measured by theromocamera (for details, see Kaňa and Vass 2008.
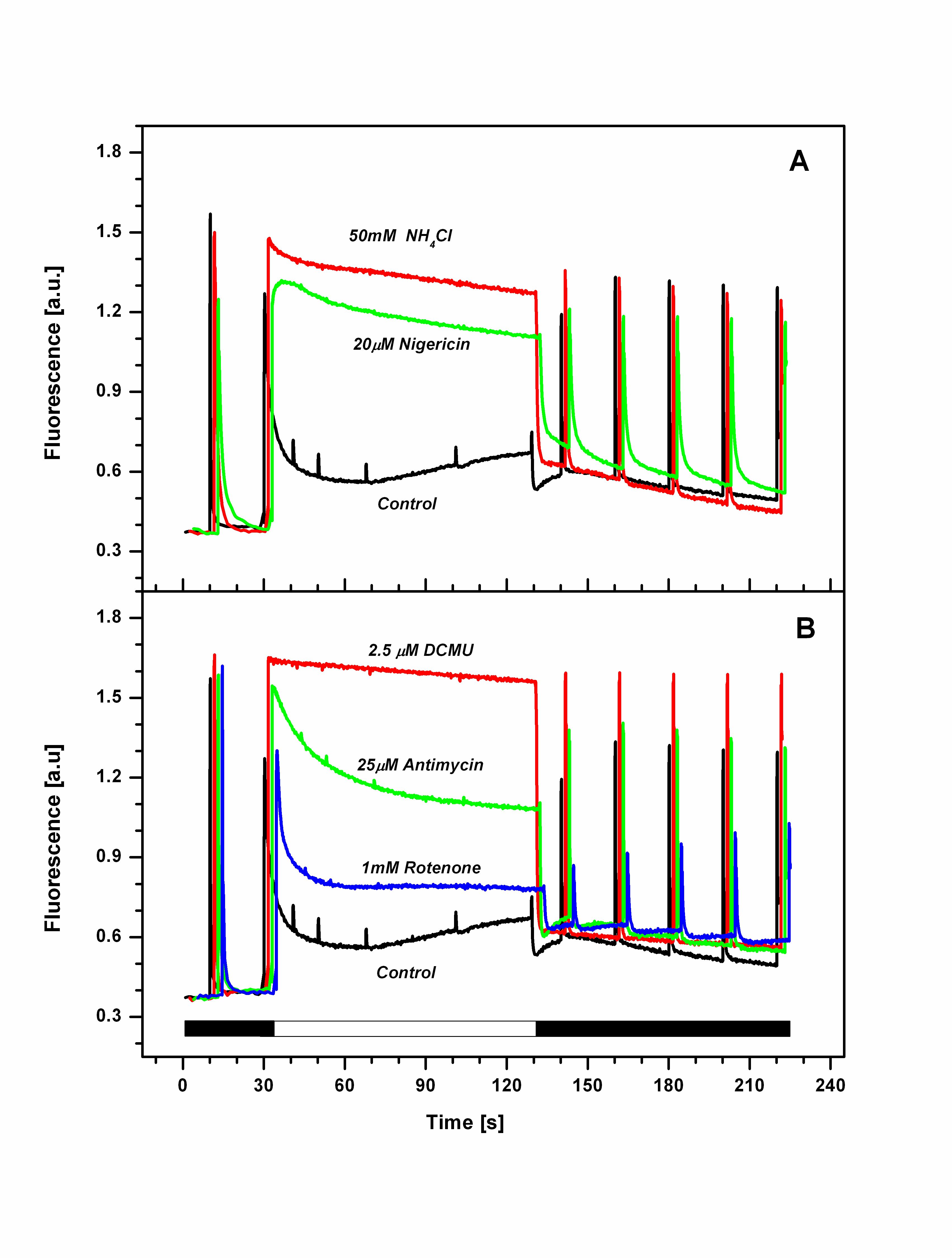
Typical quenching analysis of Rhodomonas salina cells. (for more details, see Kaňa et al. 2012)
|
||
|
Isolated antennae complexes of Rhodomonas salina cells. (for more details, see Kaňa et al. 2012) |
|||
| We focuse on mechanisms of NPQ in red-clade of photosynthetic organisms that are typical in huge variability in antennae proteins and pigment compositions. Our main model organisms are represented by Chromera velia (photosynthetic colpedelid alga) and cryptophytes (Rhodomonas salina, Guillardia theta). These algal species represent good models to study NPQ mechanisms dependent on xanthophyle cycle (C. velia - see Kotabová et al. 2011) and independent to any xanthophyll cycle (see Kaňa et al. 2012; Cheregi et al. 2015). | |||
(4) New biophysical methods in photosynthesis |
|||
|
We have adapted several new biophysical methods for photosynthesis research. It includes spectrally resolved fluorescence induction (SRFI) allowing spectral detection of fluorescence induction and fluorescence parameters (e.g. NPQ) and detection of fluorescence induction (PAM-like curves) for various chromophores simultaneously. We have also developed new method of cryoimaging (see Steinbach et al. 2015) allowing separate detection of PSII fluorescence and red shifted PSI fluorescence at 77K. |
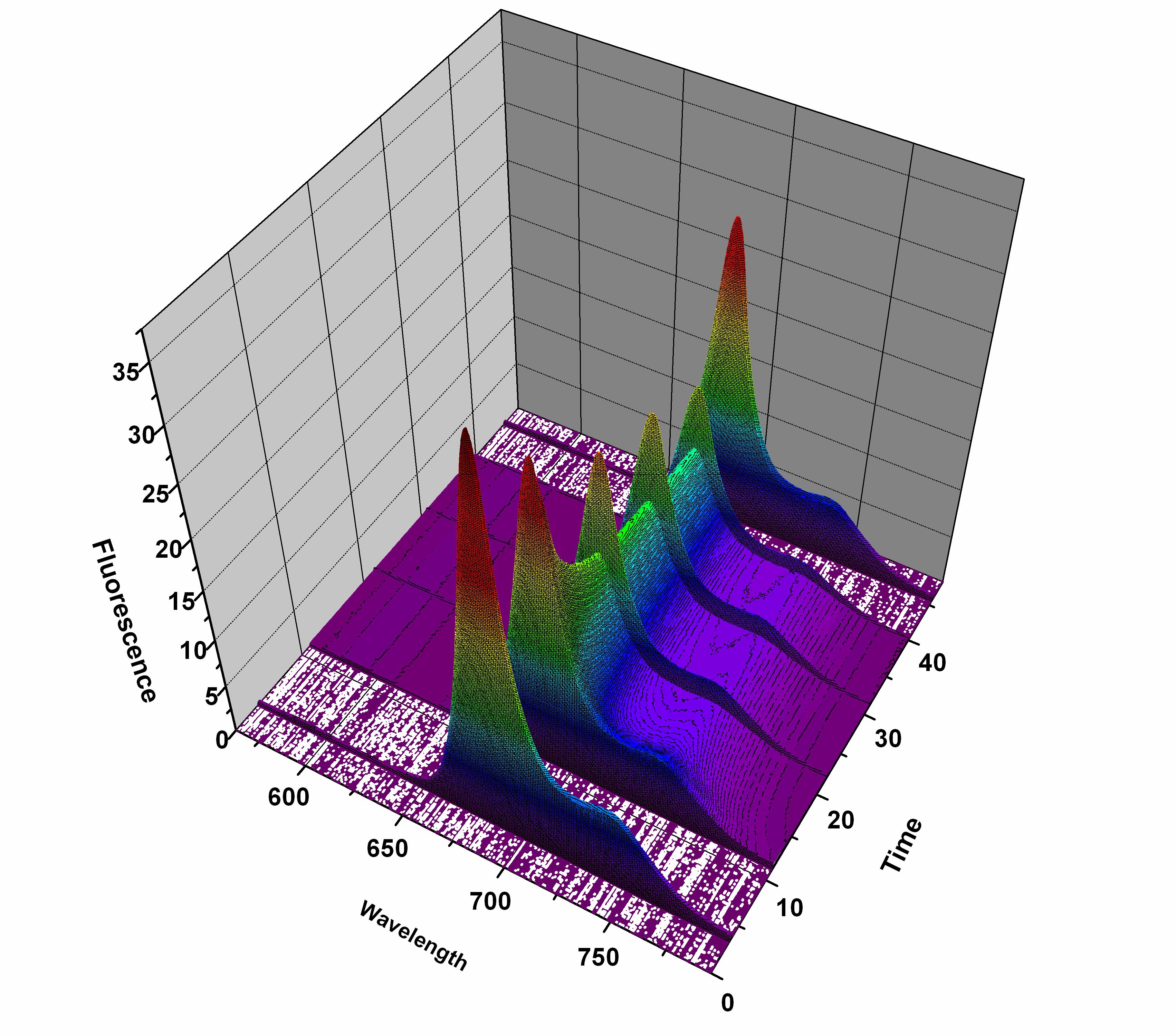
Typical time-course of Spectrally Resolved Fluorescnece Induction Method (SRFI) for cyanobacteria. |
||